abbott point of care covid test
A rapid test for the qualitative detection of COVID-19 antigens in nasal swab specimens. Patient-friendly supervised self-collected Nasal swab minimizes health worker exposure.

Point Of Care Testing Diagnostics Testing Newsroom
Food and Drug Administration FDA for the fastest available molecular point-of-care test for the.

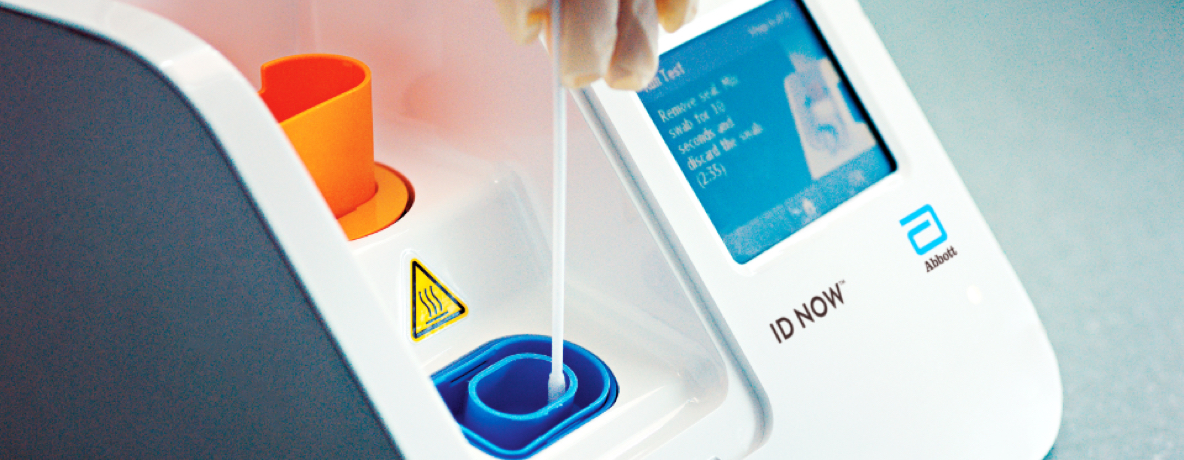
. It is used on our ID NOW platform. In addition participants with COVID-19 notified to the Victorian Government were. For more information on ID NOW check out this.
Abbott has received emergency use authorization EUA from the US. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less. The BinaxNOW COVID-19 Self Test is identical to the professional-use test used since August 2020 bringing the most studied and widely used rapid antigen test to retail shelves across the.
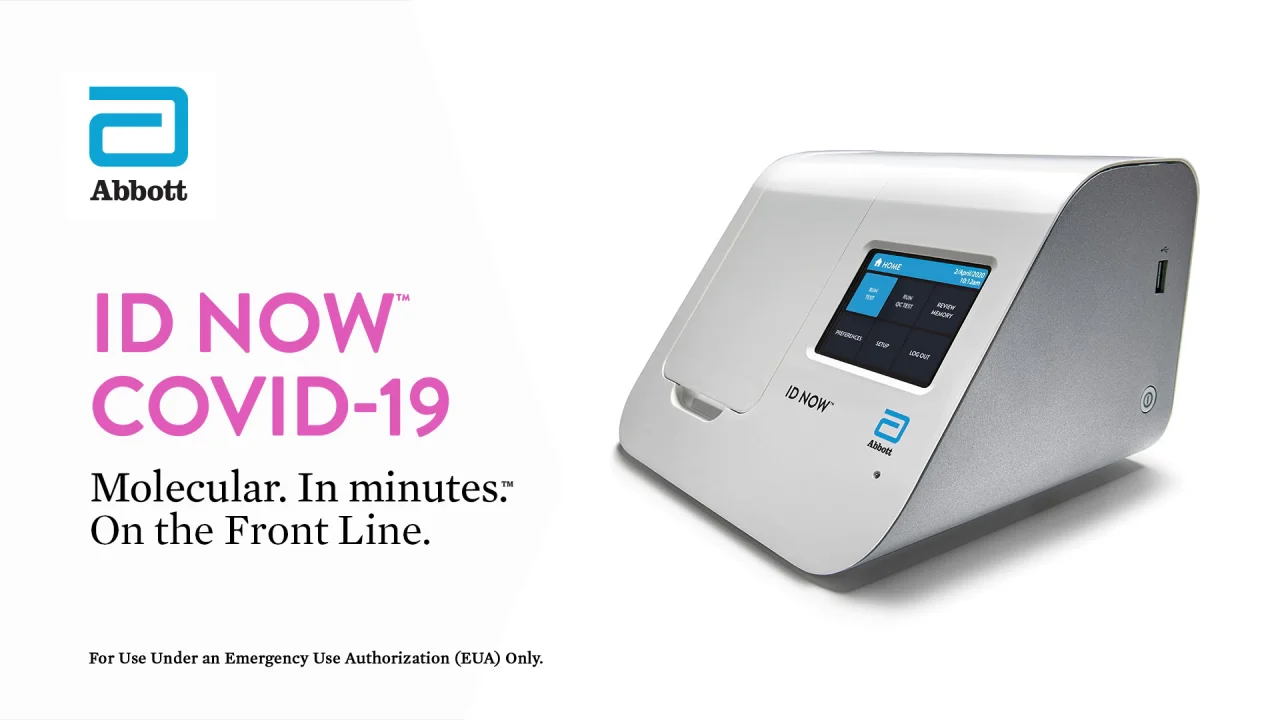
Abbotts rapid COVID-19 test isnt the only point-of-care test to receive FDA authorization during the pandemic but Trump has touted it the most by far hailing the speed at. The ID NOW COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes targeting the coronavirus COVID-19 RdRp Gene. Abbott - A Leader in Rapid.
Abbotts new point-of-care test for the novel coronavirus that causes COVID-19 was approved by the US. The revolutionary NAVICA app helps people navigate daily life in a new normal. Identify potentially contagious patients with or without symptoms in 15 minutes.
9125 L x 0938 D x 5063 H. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that. Point of care testing to diagnose and manage diabetes and its comorbidities.
The ID NOW platform combines the benefits of speed and accuracy for the fastest molecular results in the market. Our rapid molecular point-of-care test detects COVID-19 in 13 minutes or less. Diagnostics Testing May 27 2020.
The arrival of the Abbott ID NOW COVID-19 test comes a week after the company launched its Abbott m2000 RealTime SARS-CoV-2 EUA test which runs on the. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start. Food and Drug Administration FDA under Emergency Use.
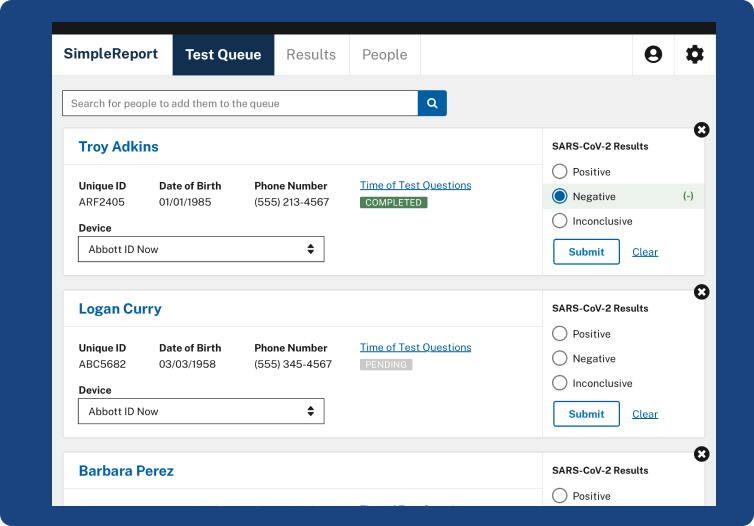
Results from the simple nasal swab are available in. Each box contains two tests for frequent serial testing and has a suggested retail price of 2399. This test is used on our ID NOW instrument.
Kit contains all necessary components for testing. Abbott is transforming care by giving people and their doctors timely information to better manage. The ID NOW COVID-19 test returns positive results in 13 minutes or.
Our ID NOW test for COVID-19 is the fastest molecular point-of-care rapid test available today and has been delivering reliable results. Taking COVID-19 Testing to a New Level. The ID NOW COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes targeting the coronavirus COVID-19 RdRp Gene.
This guidance provides information on the regulatory requirements for SARS-CoV-2 rapid testing performed in point-of-care settings collecting specimens and. NAVICA displays results from the 15-minute Abbott. Our other rapid COVID-19 test is the ID NOW system a molecular point-of.
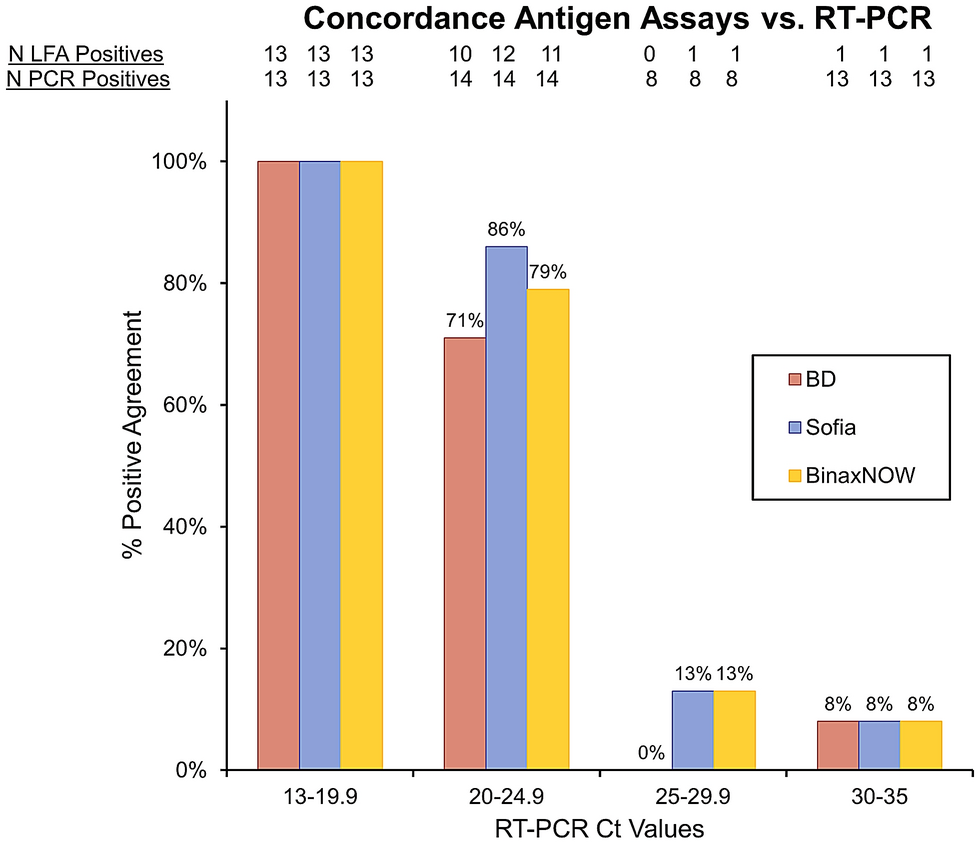
The Abbott PanBio TM COVID-19 Ag point-of-care test was performed alongside RT-PCR.

Id Now Covid 19 Abbott Point Of Care

Sars Cov 2 Testing Sensitivity Is Not The Whole Story

Maine S First Recipient Of Abbott Labs Rapid Covid 19 Test Is Martin S Point Health Care Newscentermaine Com

Laboratory Resources For Poc Reader
Multidisciplinary Assessment Of The Abbott Binaxnow Sars Cov 2 Point Of Care Antigen Test In The Context Of Emerging Viral Variants And Self Administration Scientific Reports
Abbott Launches Fastest Point Of Care Covid 19 Test On Market To Date Homeland Preparedness News
Covid 19 Testing The Center For Urgent Care

Abbott Realtime Sars Cov 2 Assay Eua Abbott Molecular

Approval Of At Home Tests Releases A Pandemic Fighting Weapon Harvard Gazette

Rapid 5 Coronavirus Test Doesn T Need Specialty Equipment Ap News

Baker Polito Administration Announces Higher Education Holiday Travel Guidance First Round Of Abbott Binaxnow Covid 19 Point Of Care Testing In K 12 Schools Mass Gov

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News

Abbott S Fast Covid Test Poses Safety Issues Lab Workers Say Kaiser Health News
Id Now Covid 19 Abbott Point Of Care

Covid 19 Rapid Tests Accuracy Types And Where To Find Them Goodrx
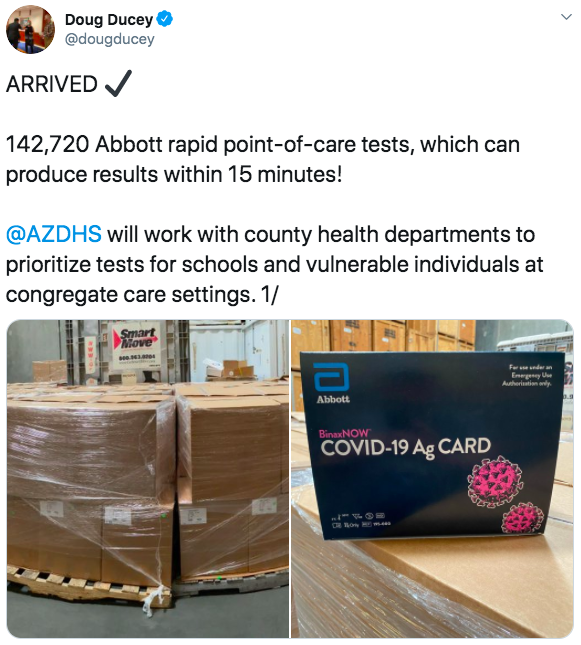
Primer Arizona Continues To Ramp Up Testing Office Of The Arizona Governor